Gemporia received a question from Gayle in Harrogate, who asked Steve why crystal glassware shouldn’t really be considered crystal at all. Real crystals are defined by having a repeating pattern of atoms, which glass does not. In this blog, we’re going to explain this a little further and show you exactly how your favourite gemstones grow in nature.
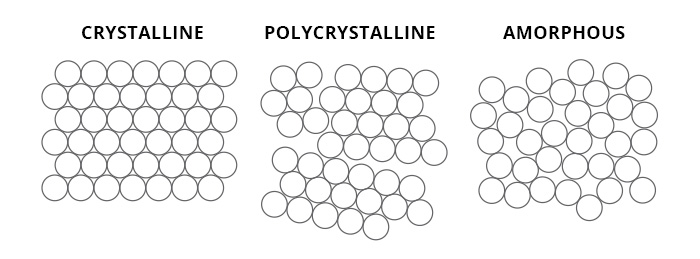
Inorganic solids are usually either amorphous or crystalline. Amorphous materials are those, like glass, which have no crystal structure. Crystals, on the other hand, have a lattice system with a recurring pattern. A true crystal’s structure can be broken down into unit cells which are the smallest unit which can be repeated throughout the crystal.
THE DISCOVERY OF CRYSTALLOGRAPHY
In 1783, a French mineralogist called René Just Haüy was working in his lab and happened to drop a Calcite crystal on the hard floor. The Calcite sample shattered into many smaller pieces. Haüy happened to notice that all of the shattered fragments, regardless of size, all had the same rhombohedral shape as the original crystal. He realised that this must mean that crystals are made up of a large number of smaller units, each of which must have an identical shape.

In 1850, Auguste Bravais discovered that there are 14 possible arrangements of atoms in crystals (more on that here). Later work by Max von Laue in 1914, proved conclusively that minerals have regular atomic patterns when he discovered the diffraction of X-rays through crystals.
WHAT IS A CRYSTAL MADE OF?
Crystals are made up of atoms of chemical elements bonded together in a recurring pattern. These can be made up of commonly occurring elements or rarer ones. Just one gemstone – Diamond, is made up of a single element – carbon.

Crystals with the same chemical composition can, however, form different gemstones. Al2SiO5 is a compound formed of two parts aluminium, one part silicon and five parts oxygen. This can form one of three different gemstones – Kyanite, Sillimanite or Andalusite – depending on how the atoms arrange themselves, which is in turn dependent on the amount of heat and pressure applied when they are forming. This is called polymorphism.
HOW DO CRYSTALS GROW?
To understand how crystals grow, we must first understand why they grow at all. This comes down to why atoms join together in the first instance.
An atom is made up of protons (which have a positive charge), neutrons (which have no charge) and electrons (which have a negative charge). The different numbers of protons in an atom dictate what element it is. The protons and neutrons collect together in the centre of the atom (which is called the nucleus). The electrons, which are attracted by the protons, are in constant motion around the nucleus. The electrons around a nucleus repel each other, counterbalancing the attraction from the nucleus, which stops the atom from collapsing in on itself.
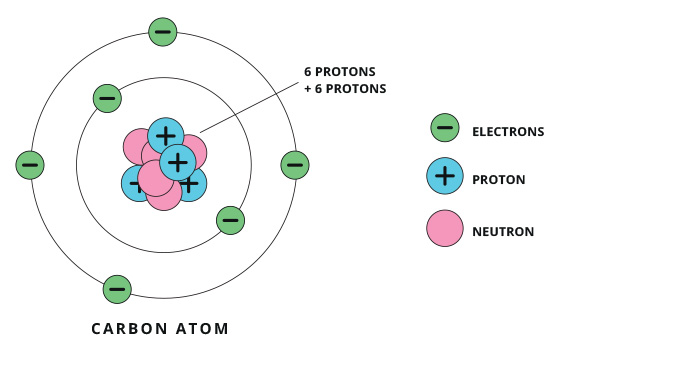
When atoms or molecules lose or gain an electron, they become charged and are then known as ions. Just like opposite ends of a magnet, ions will either attract or repel each other, depending on their charge.

When they’re liquid or gas, these ions have enough energy to overcome their attraction and slide past each other. When they become solid, the ions of opposite charges will aggregate into a lattice with each successive charged ion attracting the next nearest ion of the opposite charge. As each ion is added so each layer grows. Over time, these layers will build up in one of 14 possible patterns, eventually forming a crystal that can be extracted from the earth and faceted.
This all makes it sound like every crystal forms perfectly. But if you love your gemstones, you will be aware of the fact that gemstones aren’t perfect at all. In fact, it is their imperfections which set them apart. An Emerald wouldn’t be an Emerald without its unique character. A Dendritic Quartz would have no pattern at all!
As a crystal is growing, the electronic arrangement of the ions means that it is easier for additional ions to bind into the lattice from certain angles and slower from some others, resulting in some faces growing faster than others. Given long enough under constant and ideal conditions, crystals will grow until they are euhedral, that is, until they have perfect angular, smooth faces. However, most crystals are anhedral, meaning that they don’t have well-formed faces, usually because the growing face has met with an opposite growing face from a neighbouring crystal and there is no further room for that face to grow.

This is because crystals don’t usually have the chance to grow perfectly, they usually grow into a void (sometimes microscopic). If the space for each individual crystal to grow is very tiny, the crystal formed is called polycrystalline, meaning that there is a mass made of microscopic crystals. These minerals will look different (and have different optical properties) to a crystal which has had a large space to grow into.
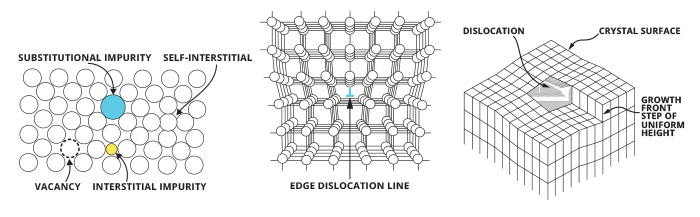
Defects in the crystal lattice are also common. Atoms can be missing (known as a vacancy), or added (known as an interstitial), or be an interloper (known as a substitution). Sometimes the crystal lattice is misaligned (known as an edge dislocation), or sometimes the lattice will form in a screw-like pattern (known as a screw dislocation). All of these changes can affect how a gemstone looks and how it performs optically.
Find your unique gemstone created by Mother Nature here..

